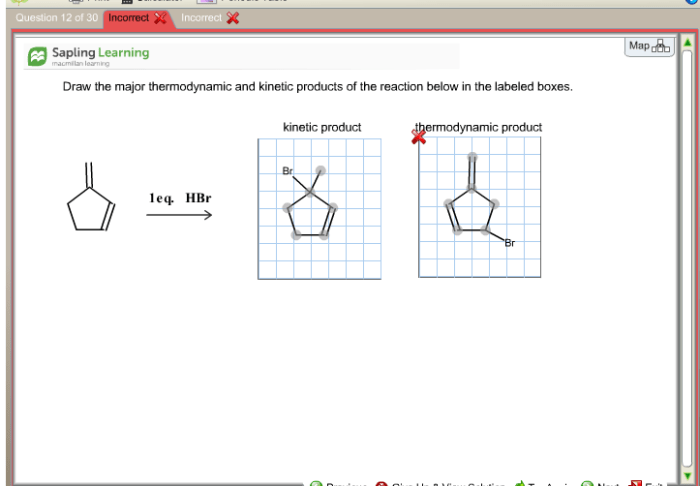
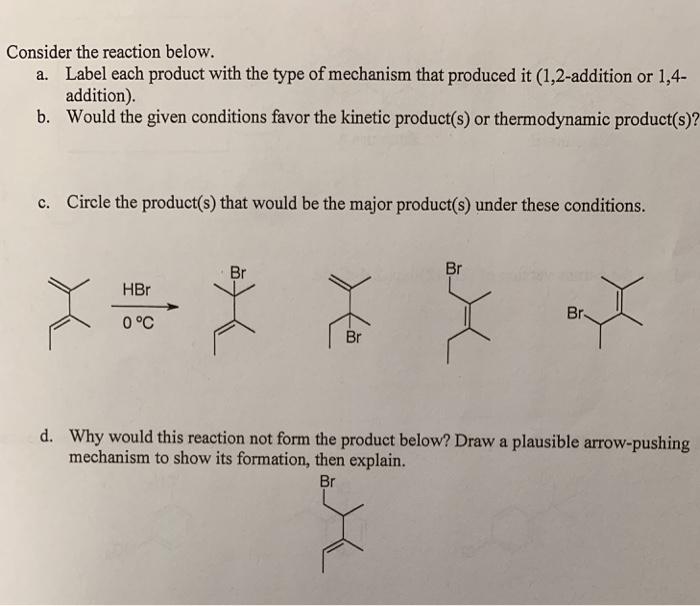
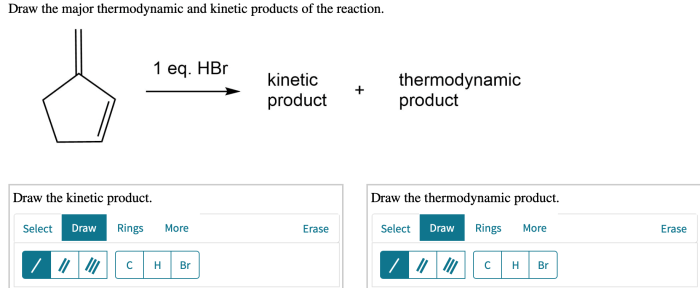
Draw the major thermodynamic and kinetic products of the reaction – Delving into the realm of reaction products, this comprehensive guide explores the intricacies of thermodynamic and kinetic products. As we embark on this journey, we will uncover their definitions, distinctions, and the factors that govern their formation. Along the way, we will delve into the fascinating world of regio- and stereoselectivity, unlocking the secrets of controlling product outcomes.
Join us as we unravel the mysteries of reaction products, empowering you with the knowledge to predict and manipulate reaction outcomes with precision.
Thermodynamic and Kinetic Products
Thermodynamic products are the most stable products that can be formed under a given set of reaction conditions. They are the products that have the lowest free energy and are therefore the most likely to form. Kinetic products, on the other hand, are the products that are formed most quickly under a given set of reaction conditions.
They are the products that have the lowest activation energy and are therefore the most likely to form initially.
The difference between thermodynamic and kinetic products can be illustrated by the following reaction:
“`A + B → C + D“`
Under thermodynamic conditions, the most stable product is C. This is because C has the lowest free energy of all the possible products. However, under kinetic conditions, the most likely product to form is D. This is because D has the lowest activation energy of all the possible products.
Factors Affecting Product Formation, Draw the major thermodynamic and kinetic products of the reaction
The formation of thermodynamic and kinetic products is affected by a number of factors, including temperature, concentration, and reaction time.
Temperature affects the formation of thermodynamic and kinetic products by changing the relative rates of the forward and reverse reactions. At higher temperatures, the forward reaction is more likely to occur, and the thermodynamic product is more likely to form.
At lower temperatures, the reverse reaction is more likely to occur, and the kinetic product is more likely to form.
Concentration affects the formation of thermodynamic and kinetic products by changing the relative amounts of the reactants. At higher concentrations, the forward reaction is more likely to occur, and the thermodynamic product is more likely to form. At lower concentrations, the reverse reaction is more likely to occur, and the kinetic product is more likely to form.
Reaction time affects the formation of thermodynamic and kinetic products by giving the reaction more time to reach equilibrium. At longer reaction times, the equilibrium constant will be reached, and the thermodynamic product will be the major product. At shorter reaction times, the kinetic product will be the major product.
Regio- and Stereoselectivity
Regio- and stereoselectivity are two important concepts in organic chemistry. Regioselectivity refers to the preference for a reaction to occur at a particular atom or group of atoms within a molecule. Stereoselectivity refers to the preference for a reaction to occur with a particular stereochemistry.
Regio- and stereoselectivity can be used to control the formation of specific products. For example, a regiospecific reaction will produce only one regioisomer, and a stereospecific reaction will produce only one stereoisomer.
Applications of Product Analysis
Product analysis is an important tool in a variety of fields, including organic chemistry, biochemistry, and pharmaceutical chemistry. Product analysis can be used to optimize reaction conditions, identify reaction mechanisms, and develop new synthetic methods.
For example, product analysis can be used to determine the optimal temperature, concentration, and reaction time for a particular reaction. Product analysis can also be used to identify the intermediates and byproducts of a reaction, which can help to elucidate the reaction mechanism.
Finally, product analysis can be used to develop new synthetic methods by identifying new ways to make specific products.
FAQ Section: Draw The Major Thermodynamic And Kinetic Products Of The Reaction
What are the key differences between thermodynamic and kinetic products?
Thermodynamic products are the most stable products, formed under equilibrium conditions, while kinetic products are formed more rapidly under non-equilibrium conditions.
How can we predict the major product of a reaction?
The major product can be predicted based on factors such as the stability of the products, the reaction conditions, and the presence of catalysts.
What is the role of regio- and stereoselectivity in controlling product formation?
Regio- and stereoselectivity allow for the selective formation of specific isomers, enabling precise control over the molecular structure of the products.